Periodic Table And Element Structure; Informative Awnsers / Molecular structure of all periodic table element ... / Interactive periodic table showing names, electrons, and oxidation states.
Periodic Table And Element Structure; Informative Awnsers / Molecular structure of all periodic table element ... / Interactive periodic table showing names, electrons, and oxidation states.. Sendus your suggestions so we can make it better. Modern version of the periodic table of the elements. Atoms are made up of protons, neutrons, and electrons. Interactive periodic table showing names, electrons, and oxidation states. A table where all the elements in existence are arranged together in rows, in order of their atomic numbers is called the periodic table.
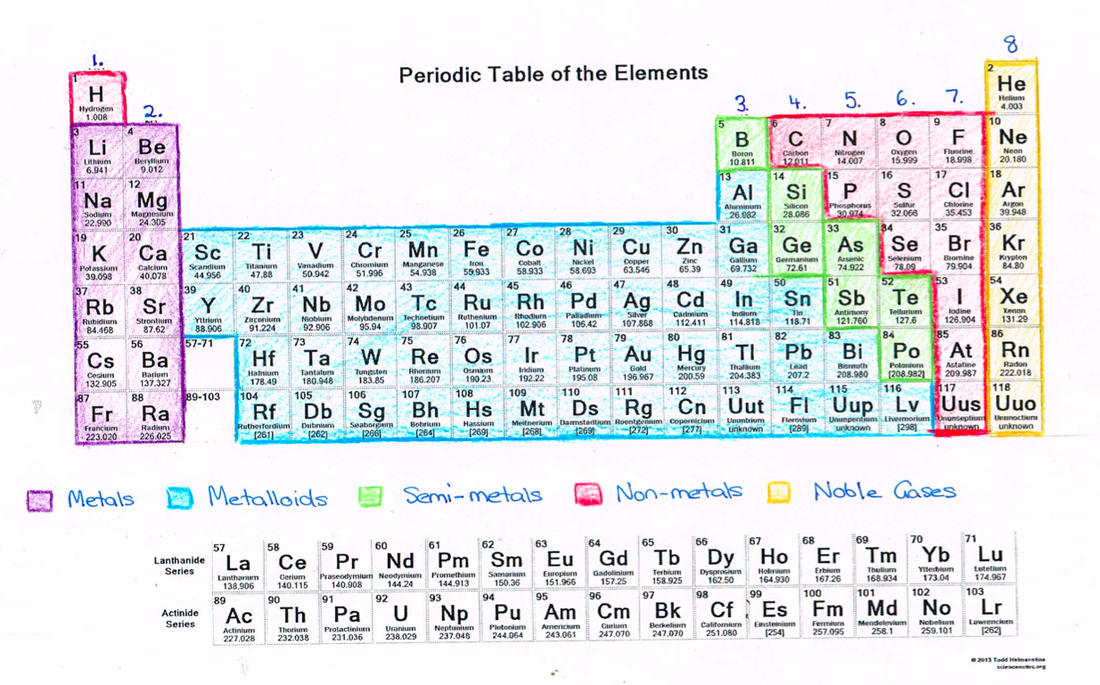
The periodic table of elements contains useful information. The periodic table contains informative cells for each element arranged by increasing atomic number and chemical properties. Each element's cell typically contains lots of. Elements in the same group have the same number of electrons in their outer shell (also called the valence electrons). The standard form of the periodic table shown here includes periods (shown horizontally) and groups (shown vertically).
The arrangement of these elements is in a grid or matrix all the elements in the period have the same number of shells.
The periodic table is a way to organize the elements based on their similarities. He put the elements into a grid with increasing atomic weights and noticed the elements in each column had similar chemical properties. The letters in each block represent the atomic symbol. The first version was constructed by dmitri mendeleev in 1869. The periodic table showing electron shellsthe elements in this table are laid out in the standard configuration of periods and groups. Iowa core content standard correlation the attraction of the electrons to the nucleus is the basis of the structure of the atom, thus each element has a unique structure arising from the interactions between electrons and their nuclei. The periodic table of elements contains useful information. Because now, the periodic table has arranged the elements according to approximate atomic number. The periodic table contains informative cells for each element arranged by increasing atomic number and chemical properties. Periodic table and element structure. Remember, the atomic number, is the number of protons. Interactive periodic table with element scarcity (sri), discovery dates, melting and boiling points, group, block and period information. Sendus your suggestions so we can make it better.
Because now, the periodic table has arranged the elements according to approximate atomic number. Each have the s and p sublevels of their outermost energy level filled. Here is how read the display to understand the elements. The periodic table is a way to organize the elements based on their similarities. The position of an element provides information about its properties.

A russian scientist called dmitri mendeleev produced one of the first practical periodic tables in the.
Interactive periodic table with element scarcity (sri), discovery dates, melting and boiling points, group, block and period information. He put the elements into a grid with increasing atomic weights and noticed the elements in each column had similar chemical properties. The first version was constructed by dmitri mendeleev in 1869. A simple overview of states of matter, atoms, electrons, protons and neutrons. The periodic table of elements arranges all of the known chemical elements in an informative array. Similar atomic numbers mean that the elements have similar atomic structure, thus similar chemical properties. Each box includes representations of the electron shell structure for the element. The number of electrons in this last shell increase by one across any given period. The periodic table gives the names and symbols of all the elements. The periodic table showing electron shellsthe elements in this table are laid out in the standard configuration of periods and groups. The physical and chemical properties of the elements are a periodic function of their atomic masses. You can see a simple version of the modern periodic table in the figure below. A periodic table is still used today to organize the elements.
Each have the s and p sublevels of their outermost energy level filled. Iowa core content standard correlation the attraction of the electrons to the nucleus is the basis of the structure of the atom, thus each element has a unique structure arising from the interactions between electrons and their nuclei. The number of electrons in this last shell increase by one across any given period. Remember, the atomic number, is the number of protons. For the element helium (shown above) the atomic number is two.

The position of an element provides information about its properties.
Iowa core content standard correlation the attraction of the electrons to the nucleus is the basis of the structure of the atom, thus each element has a unique structure arising from the interactions between electrons and their nuclei. This is essentially an abbreviation of the elements name. A periodic table is still used today to organize the elements. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! The physical and chemical properties of the elements are a periodic function of their atomic masses. The first version was constructed by dmitri mendeleev in 1869. So the periodic table of elements has been arranged in increasing order of their atomic numbers. To learn an element's name, atomic number, electron configuration, atomic weight, and more, select one from the table. Periodic table and element structure. The royal society of chemistry's interactive periodic table features history, alchemy, podcasts, videos, and data trends across the periodic table. Elements in the same group have the same number of electrons in their outer shell (also called the valence electrons). Notes actinides and lanthanides are collectively known as rare earth elements. The periodic table of elements arranges all of the known chemical elements in an informative array.
Komentar
Posting Komentar